Post written by Taylor Bowler, DO, from the Department of Internal Medicine, Rahul Karna, MD, from the Department of Gastroenterology, Hepatology and Nutrition, University of Minnesota Medical Center, Minneapolis, Minnesota, and Mohammad Bilal, MD, from the Division of Gastroenterology & Hepatology, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.

Our article highlights a case of an 85-year-old man with multiple comorbidities who presented with a 60-mm persistent recurrent polyp adjacent to the ileocecal valve despite multiple prior resection attempts. The polyp was successfully removed with multistaged endoscopic full-thickness resection (EFTR) using the full-thickness resection device (FTRD) (Ovesco Endoscopy AG, Tübingen, Germany).
Initial resection attempts were made using the piecemeal cold snare endoscopic mucosal resection technique followed by endoscopic resection using the endoscopic powered resection device in combination with hot avulsion and hybrid argon plasma coagulation. Polyp histopathology was consistent with a tubulovillous adenoma (TVA) with high-grade dysplasia (HGD).

On surveillance colonoscopy, a 60-mm lesion was noted at the same location. The decision was made to attempt multistaged EFTR with the FTRD in a piecemeal fashion given the patient was deemed a poor surgical candidate, as well as other features such as large lesion size, technically challenging lesion location, and recurrence despite prior use of different modalities.
A total of 3 colonoscopies with EFTR using the FTRD were performed over 14 months with successful removal of the recurrent polyp. Histopathology showed TVA with HGD after the first resection and TVA without HGD on the last 2 resections with negative deep margins on all specimens. Subsequent surveillance colonoscopy after the last resection showed no evidence of residual or recurrent polyp.

Although several options are available for management of large recurrent polyps including repeat endoscopic mucosal resection with hybrid argon plasma coagulation or hot/cold avulsion, endoscopic submucosal dissection, endoscopic resection using the endoscopic powered resection device, and EFTR using the FTRD, each technique bears its own limitations with regard to lesion size, anatomic location, and adverse events. To our knowledge, few articles have outlined the use of multistaged piecemeal EFTR using the FTRD as an alternative modality for management of large recurrent polyps in challenging anatomical locations.
Furthermore, this intervention may offer a safe solution in select patients who are deemed poor surgical candidates because of medical comorbidities or patients with advanced age who would prefer to avoid surgery altogether. Future studies should compare the use of multistaged EFTR using the FTRD with conventional modalities for management of large recurrent polyps.
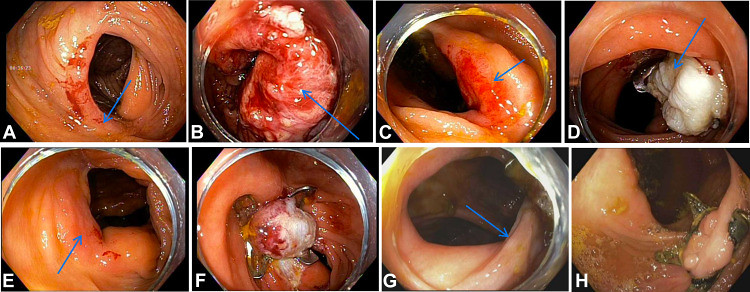
A, Recurrence of 60-mm polyp (arrow) on surveillance colonoscopy. B, Resection site (arrow) after use of the endoscopic-powered resection device (EndoRotor, Interscope Inc, Northbridge, Mass, USA). C, Recurrence of 60-mm polyp (arrow) on surveillance colonoscopy after use of the device. D, Resection site with clip (arrow) after first endoscopic full-thickness piecemeal resection. E, Residual polyp (arrow) before second endoscopic full-thickness piecemeal resection. F, Resection site after second endoscopic full-thickness piecemeal resection. G, Residual polyp (arrow) before third endoscopic full-thickness piecemeal resection. H, Resection site with no recurrence on surveillance colonoscopy after multistaged endoscopic full-thickness piecemeal resection.
Visit iGIE’s Facebook, X/Twitter, LinkedIn, Instagram, and YouTube accounts for more content from the ASGE peer-reviewed journal that launched in December 2022 and has been PubMed indexed.
Read the full article online.
The information presented in Endoscopedia reflects the opinions of the authors and does not represent the position of the American Society for Gastrointestinal Endoscopy (ASGE). ASGE expressly disclaims any warranties or guarantees, expressed or implied, and is not liable for damages of any kind in connection with the material, information, or procedures set forth.
